1 The starting point of electronic equipment
Silicon is the 14th element in the periodic table. It is one of the basic components of the universe. Protons are heavier than aluminum and protons are lighter than phosphorus. However, silicon often appears. It is the main component of the building materials that make up everyone's houses and the basis of all current computer processors. The most important of the unique features of silicon is that it is simple, and many of them are hell. After oxygen, it is the second most abundant element in the earth's crust-but don't expect it to be everywhere. In nature, silicon has almost never been found in a pure state. In fact, silicon is always mixed with other elements. The most common is silicate or silica. Silica is a coarse and highly polluted form and is the main component of sand. Feldspar, granite, quartz, etc. are all based on silica compounds.
2 Silicon original
Silicon compounds have many useful properties, mainly because they can bond other atoms very tightly and in complex arrangements. Various silicates such as calcium silicate are the main components of cement and the main binder in cement, mortar and even plaster. Certain silicate-rich materials can be heated to produce hardened ceramics, such as porcelain, while others can be fused to form the world’s main glass soda lime glass. Silicon can also be used as a trace additive in other substances such as cast iron, which uses both carbon and silicon to make the iron more elastic and less brittle. Moreover, silicon is also the main structural component of synthetic material silicone resin, but do not confuse the two-if it is indeed Silicon Valley, then the technological world is very different from where we see it today.
When selecting the components used as the basis of computer transistors, the key word is resistance. Conductors have low resistance and are very easy to pass current, while insulators have (predictably) high resistance that can slow down or stop the flow of electrons. For transistors that must be able to be turned on and off at will, we need a substance with a resistance between a conductor and an insulator in a semiconductor. According to needs, various "dopants" can be used to process the best semiconductors in the industry to fine-tune their resistance.
3 Semiconductor material

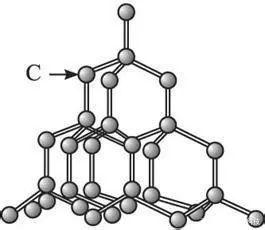
Silicon is not the only semiconductor material on earth, or even the best semiconductor on earth. It is by far the most abundant semiconductor on earth. Silicon is easily available all over the world. You don't need to import it from special mines in Africa, and you don't need to spend months of expensive and pollution treatment to get some. It is easy to operate, and most importantly, scientists have proposed a reliable method to grow it into an ordered crystal. These crystals are to silicon what diamond is to carbon. Growing huge, near-perfect silicon crystals is one of the main skills in modern computer chip manufacturing. These crystals are then cut into thin slices, which are then carved, processed and processed in hundreds of different ways, then cut into small pieces and packaged in commercial processors. It is possible to use exotic materials such as carbon and germanium to make high-quality transistors, but none of them can achieve mass production of silicon through the growth of large crystals, at least not yet.
4 Why use silicon

Currently, silicon crystals are made in cylinders with a diameter of 300 mm, but research is rapidly approaching the 450 mm threshold. This will help reduce production costs, so that the speed will continue to increase for at least a decade or so. In the end, there may be no choice but to abandon silicon. Of course, since there is more silicon on earth than carbon, there must be a reason that we are organic carbon-based instead of silicon-based, and this reason is back in the periodic table. Without much discussion, the lower vertical elements in the periodic table have heavier nuclei and larger electron shells. Silicon is physically larger and heavier than carbon, making it less suitable for ultra-fine tasks such as recombinant DNA. Silicon is also less reactive than carbon, which means that the chemical diversity of silicon-based life may be lower, or a wider range of reactions may be required to drive silicon enzymes to force the presence of chemically undesirable compounds.
5 What to do if you run out
Although there are almost thousands of silicon atoms on Earth than carbon atoms, all life on Earth is organic, which may indicate the possibility of this possibility occurring elsewhere in the universe. There are many species here that use silicon to some extent, but none of them use silicon as a structural element of DNA. Life on the basis of silicon is certainly possible, but if it does exist, it is likely that it will never develop to the level of sophistication that carbon allows at home. For many years to come, silicon will continue to appear in everyone's news feeds. Even if some people regard carbon and other non-silicon elements as the platform for next-generation computing, it will be essential if we continue to maintain the historical trend of computing power, but silicon is still the first choice in many fields.



 Account not verified
Account not verified