Graphite is an allotrope of carbon. It is a gray-black, opaque solid. It has stable chemical properties, corrosion resistance, and is not easy to react with acids, alkalis and other chemicals. It burns in oxygen to generate carbon dioxide, which can be oxidized by strong oxidants such as concentrated nitric acid and potassium permanganate . It can be used as an anti-wear agent and lubricant. High-purity graphite is used as a neutron moderator in atomic reactors. It can also be used to make crucibles, electrodes, brushes, dry batteries, graphite fibers, heat exchangers, coolers, electric arc furnaces, Arc lights, pencil refills, etc.

graphite
Alias
Shiniu, Shihei, Shiluo, Shidai, Thrush
Chemical formula
C
Molecular weight
12.01
CAS Registry Number
7782-42-5
EINECS registration number
231-955-3
Melting point
3652 to 3697 ℃
Boiling point
4830 ℃
Water soluble
not soluble in water
Density
2.09 to 2.33 g/cm³
Exterior
Black solid
Application
Pencil lead, refractory materials, conductive materials, lubricating materials, carbon manufacturing, radiation protection materials, etc.
Security description
S22;S26;S36;S36/S37
Hazard symbol
R20;R36/37
Risk description
Non-toxic, dust inhalation can cause respiratory diseases
Chemical nature
Stable, corrosion-resistant, not easy to react with chemicals such as acids and alkalis
Introduction
Structure and composition
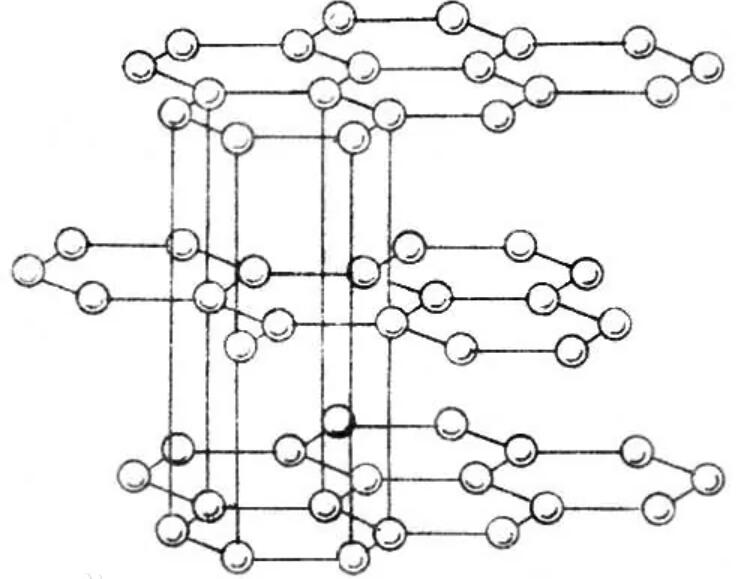
Graphite is a transition type crystal between atomic crystals , metal crystals and molecular crystals. In the crystal, the carbon atoms in the same layer are hybridized with sp 2 to form a covalent bond. Each carbon atom is connected to three other carbon atoms. The six carbon atoms form a regular hexagonal ring on the same plane and stretch to form a sheet. structure. The carbon atoms in the same plane also have a p-orbital each, which overlap each other to form a delocalized π-bond electron that can move freely in the lattice and can be excited, so graphite has metallic luster, can conduct electricity and transfer heat. Due to the large distance between the layers, the small bonding force ( Van der Waals force ), and the sliding of each layer, the density of graphite is smaller than that of diamond , and it is softer and has a slippery feel.
Molecular structure (1 photo)
The distance between each layer of graphite is 3.40Å, which is combined by van der Waals forces, that is, the layers are molecular crystals. The distance between carbon atoms in the same layer is 1.42Å, due to the carbon atoms on the same plane layer. The intermolecular bond is very strong and extremely difficult to break, so the melting point of graphite is also very high, and the chemical properties are also stable. In view of its special bonding method, it cannot be regarded as single crystal or polycrystal alone. Now it is generally considered that graphite is a kind of mixed crystal.Graphite belongs to the hexagonal crystal system with complete layered cleavage. The cleavage surface is dominated by molecular bonds, which has weaker attraction to molecules, so its natural floatability is very good.
Physical and chemical properties
Graphite is soft, dark gray, greasy, and can contaminate paper. The hardness is 1-2, and the hardness can increase to 3-5 with the increase of impurities in the vertical direction. The specific gravity is 1.9 to 2.3. The specific surface area is concentrated in the range of 1-20m 2 /g. In the absence of oxygen, its melting point is above 3000 ℃, and it is one of the most temperature-resistant minerals. It can conduct electricity and heat.
There is no pure graphite in nature, and it often contains impurities such as SiO 2 , Al 2 O 3 , FeO, CaO, P 2 O 5 , and CuO. These impurities often appear in the form of minerals such as quartz, pyrite, and carbonate. In addition, there are water, asphalt, CO 2 , H 2 , CH 4 , N 2 and other gas components. Therefore, in the analysis of graphite, in addition to determining the fixed carbon content, the volatile and ash content must also be determined at the same time .
Graphite and diamond , carbon 60 , carbon nanotubes , graphene, etc. are all simple substances of carbon, and they are allotropes .
Special nature
Due to its special structure, graphite has the following special properties:
(1) High temperature resistance
The melting point of graphite is 3850±50°C. Even after ultra-high temperature arc burning, the weight loss is very small and the thermal expansion coefficient is also very small. The strength of graphite increases with the increase of temperature. At 2000°C, the strength of graphite doubles.
(2) Electrical and thermal conductivity
 graphite
graphite(3) Lubricity
The lubricating performance of graphite depends on the size of the graphite flakes. The larger the flakes, the smaller the friction coefficient and the better the lubricating performance.
(4) Chemical stability
Graphite has good chemical stability at room temperature, and is resistant to acid, alkali and organic solvent corrosion.
(5) Plasticity
Graphite has good toughness and can be crushed into very thin flakes.
(6) Thermal shock resistance
Graphite can withstand drastic changes in temperature without being damaged when used at room temperature. When the temperature changes suddenly, the volume of graphite does not change much, and cracks will not occur.
other
Graphite can be divided into two categories: natural graphite and artificial graphite. Natural graphite comes from graphite deposits. Natural graphite can also be divided into flake graphite, earthy graphite and block graphite. The graphite obtained from natural mining contains a lot of impurities, so it needs beneficiation to reduce its impurity content before it can be used. The main purpose of natural graphite is to produce refractory materials, electric brushes, flexible graphite products, lubricants, lithium ion battery anode materials, etc. Some carbon products sometimes add a certain amount of natural graphite.
The largest production volume in the carbon industry is various artificial graphite products. Artificial graphite products generally use easily graphitized petroleum coke and pitch coke as raw materials, and undergo batching, kneading, molding, roasting, graphitization (high temperature heat treatment) and mechanical processing. After a series of processes, the production cycle is as long as dozens of days.
Graphite (12 sheets)
There are many types of artificial graphite, such as single crystal graphite, polycrystalline graphite, pyrolytic graphite, highly oriented pyrolytic graphite, polyimide synthetic graphite, graphite fiber, etc. Most artificial graphite products belong to the category of polycrystalline graphite. The main products of artificial graphite are graphite electrodes used in electric arc steelmaking furnaces and submerged electric furnaces. Graphite electrodes are a high-temperature and corrosion-resistant conductive material. Artificial graphite also has a wide range of uses in many other industrial sectors, such as electrical brushes, precision casting molds, EDM molds and wear-resistant parts in the machinery industry, electrical conductors or corrosion-resistant equipment used in electrolytic cells in the chemical industry, High-purity and high-strength artificial graphite is a structural material for reactors in the nuclear industry and parts for missiles and rockets. Graphite can also be made into heat dissipation materials, sealing materials, heat insulation materials, and radiation protection materials. Graphite functional materials are widely used in metallurgy, chemical industry, mechanical equipment, new energy vehicles, nuclear power, electronic information, aerospace and defense industries. The European Commission’s "Raw Materials Vital to the EU’s Life and Death" report listed graphite among 14 scarce mineral raw materials.
Distribution and classification of minerals
Mineral distribution
The large and medium-sized graphite deposits discovered in the world are mainly distributed in China, India, Brazil, Czech Republic, Canada, Mexico and other countries. According to the US Geological Survey, the world's graphite reserves are 71 million tons, and China's graphite reserves are 55 million tons, accounting for 77% of the world. Brazilian graphite mines are distributed in Minas Gerais, Ceara and Bahia, and the best graphite is distributed in Paidrayajur, Minas Gerais ( Pedra Azul), the proven ore reserves are 250 million tons. Indian graphite mines are mainly distributed in the states of Orissa and Rajasthan. According to the "Annual Report of Indian Mining", India has 10.75 million tons of graphite reserves and 158.025 million tons of resources. Canadian graphite deposits are distributed in Ontario, British Columbia and Quebec. The Bissett Creek graphite deposit is the largest graphite deposit in North America. Sri Lanka's veined graphite deposits are world-famous and are the only highly graphitized veined graphite deposits in the world, located in the west and southwest of the island of Sri Lanka.
There are two types of graphite minerals in China: crystalline graphite and cryptocrystalline graphite. According to statistics from the Ministry of Land and Resources, as of the end of 2009, China's crystalline graphite mineral reserves were 30.41 million tons, the basic reserves were 54.32 million tons, and the resources were 13.54 million tons. In the past 20 years, my country's crystalline graphite reserves have been increasing, but the reserves of large-scale high-quality graphite have decreased to less than 5 million tons. Crystalline graphite is distributed in 20 provinces (autonomous regions) including Heilongjiang, Shandong and Inner Mongolia.
Resource classification
Graphite deposits are mainly medium and small, and the deposit types are roughly divided into the following five types: ①Lamellar graphite deposits in crystalline schist; ②Graphite deposits in metamorphic coal seams; ③Graphite deposits in nepheline syenite; ④Silicon Graphite deposits in karite; ⑤ Vein graphite deposits in crystalline schist.
There are three types of natural graphite resources, which are block graphite, flake graphite and earthy graphite (cryptocrystalline graphite).
(1) Dense crystalline graphite
Dense crystalline graphite is also called massive graphite. Such graphite crystals are clearly visible to the naked eye. The particle diameter is greater than 0.1 mm, the specific surface area is concentrated in the range of 0.1-1m/g, and the crystal arrangement is disorderly and presents a dense block structure. This kind of graphite is characterized by high grade, generally the carbon content is 60-65%, sometimes 80-98%, but its plasticity and smoothness are not as good as flake graphite.
Lump graphite is the rarest and most valuable graphite mine, mainly found in Sri Lanka.
(2) Flake graphite
Flake graphite is a combination of many single layers of graphite. It exists as a single flake in metamorphic rocks. It has a small reserve and high value. The crystals are scaly, which are metamorphic under high-intensity pressure. The difference between scales and fine scales. The characteristic of this type of graphite ore is that the grade is not high, generally between 2 to 3%, or 10 to 25%. It is one of the best floatable ore in nature, and high-grade graphite concentrate can be obtained through multiple grinding and multiple selection. The floatability, lubricity, and plasticity of this type of graphite are superior to other types of graphite, so it has the greatest industrial value.
Flake graphite is mainly distributed in Australia, Brazil, Canada, China, Germany and Madagascar. In recent years, a large number of flake graphite resources have also been discovered in Africa, Tanzania and Mozambique. Some scholars have studied flake graphite ore in Ancuaba, Mozambique and Chilalo, Tanzania. The results show that the mineral composition of graphite ore in Ancuaba and Chilalo is similar, and they are all high-quality large flake graphite resources.
(3) Cryptocrystalline graphite
Cryptocrystalline graphite is also called microcrystalline graphite or earthy graphite. The crystal diameter of this kind of graphite is generally less than 1 micron, and the specific surface area is concentrated in the range of 1-5m/g. It is an aggregate of microcrystalline graphite and can only be used under an electron microscope. See crystal form. This type of graphite is characterized by its earthy surface, lack of luster, and its lubricity is slightly worse than that of flake graphite. Higher grade. Generally 60-85%, a few as high as 90%. Generally used in the foundry industry. With the improvement of graphite purification technology, the application of earthy graphite has become more and more extensive.
Earthy graphite is the one with the most reserves. It has small scales and low crystallinity. It is used to produce low-value products. It is the lowest price among the three graphites. Earthy graphite is mainly stored in Turkey, China, Europe, Mexico and the United States.
Global graphite reserves statistics in 2015 are 230 million tons, of which Turkey’s graphite reserves are 90 million tons, accounting for 39.13% of global reserves, Brazil’s 72 million tons, accounting for 31.30% of global reserves, and China’s 55 million tons, accounting for global reserves. Of 23.91%.
Types of | Earthy graphite | Flake graphite | Block graphite |
Crystalline state | not good | better | well |
Crystal size | 0.01~0.1μm | 0.05~1.5 mm (>1.0 μm) | >0.1 mm |
grade | 60%~80%, a few as high as above 90% | 2%~5%, or between 10%~25% | 60%~65%, up to 80%~98% |
Floatability | not good | it is good | it is good |
Origin | Turkey, Europe, China, Mexico and the United States | Australia, Brazil, Canada, China, Germany and Madagascar | Sri Lanka |
Industry Overview
Overview of mineral development
In 2010, the world's natural graphite production was 1.1 million tons. China's graphite production is 800,000 tons (crystalline graphite and cryptocrystalline graphite), accounting for 73% of the world's production. In the past 30 years, China's graphite output has ranked first in the world. In 1995, China's graphite production reached the highest level in history, at 2.215 million tons, of which 549,000 tons of crystalline graphite and 1.656 million tons of cryptocrystalline graphite. In 2008, the output of crystalline graphite in China hit a record high of 650,000 tons; in 2009, the output of crystalline graphite fell to 480,000 tons, and the output of earthy graphite ore was about 1 million tons in the same period. China's crystalline graphite is developed in 16 provinces (autonomous regions) including Inner Mongolia, Heilongjiang, Shandong, Hebei, Henan, Hubei, and Sichuan. Important production areas are Jixi and Luobei in Heilongjiang, Pingdu and Laixi in Shandong, Xinghe in Inner Mongolia, Chicheng in Hebei, etc. Neixiang in Henan, Yichang in Hubei and Nanjiang in Sichuan. The development of cryptocrystalline graphite in China is mainly in the Chenzhou area of Hunan Province and the Panshi area of Jilin Province. There are many companies in the Chenzhou area of Hunan Province that use ultra-high temperature technology to produce high-purity microcrystalline graphite.
India's graphite output ranks second in the world, accounting for 11.6% of the world's graphite output. India's graphite output in 2009 was 130,000 tons. Graphite development is mainly in Orissa and Rajasthan. Orissa’s graphite production accounts for 65% to 75%. The main producer is Agrawal Graphite Industries. The company has Ganjaudar and Thai Temtimal has two graphite mines; TP Minerals Company develops graphite mines near Phulbani, Madagudarf and Sargipali. The two companies produce graphite flakes and Powder graphite products. Agrawal Graphite Industries plans to develop new graphite products for the battery industry.
Brazil's graphite production ranks third in the world, accounting for 7.5% of the world's graphite production. Graphite production has been stable in recent years, and the output of graphite in 2009 was 76,000 tons. Brazil National Graphite Co., Ltd. (National de Grafite) is the country’s main graphite producer and one of the world’s largest producers of natural crystalline graphite. It has 3 crystalline graphite mines in Minas Gerais, The graphite production capacity is 52,000 tons/year, which accounts for about 2/3 of Brazil’s total output; each mine has a graphite processing plant, the carbon content of the raw ore of ltaperica graphite ore is 16%, and the processing plant’s production capacity At 14,400 tons/year, graphite products are used in batteries, brushes and lubricants; the Salto de Divsa graphite processing plant produces large scale graphite, which are used in refractory materials, crucibles, metallurgical molds and The production capacity of high-carbon steel additives has been expanded to 14,400 tons/year; Pedra Azul is the largest graphite deposit in Brazil, with a graphite production capacity of 45,800 tons/year. Brazil National Graphite Co., Ltd. plans to adjust the structure of graphite products and develop and produce new products of spheroidized graphite for use in the battery industry, including large lithium-ion batteries for electric vehicles. Another important graphite producer in Brazil is Grafita MG Ltd., which also mines graphite in the Minas Gerais region. Mag-nesita, a Brazilian refractory company, plans to develop graphite minerals in eastern Brazil. The graphite production capacity will reach 40,000 tons/year within 2 years. The company's development will expand to upstream mineral raw materials, with the goal of ensuring self-sufficiency in the supply of graphite raw materials.
Canada's graphite production is 25,000 tons, and the main graphite producers are Timcal and Eagle Graphite. Timcal Canada’s mine and processing plant are located in Lac des Iles, and its production capacity is kept secret. In 2007, many Canadian developers planned to build graphite production bases. Due to the financial crisis, the graphite development projects of these companies were suspended. In 2010, the demand for graphite in the international market increased and the price of graphite rose. It is estimated that the new graphite development projects planned in Canada will start construction one after another. Industrial Minerals Corporation (IMI) developed the Bissett Creek (Bissett Creek) flake graphite mine, located in Mary City, Ontario. The company's goal is to become the largest flake graphite producer in North America. Fortune Graphite Inc. develops cryptocrystalline graphite and flake graphite. The mine is located in the Kootenay area of southeastern British Columbia. Quinto Mining Corp. developed the LacGueret graphite deposit, located in the Cotenord region of northeastern Quebec. The graphite grade of the deposit is 15% to 40%. Global Graphite Co., Ltd. developed Superior graphite deposit, which is flake graphite with 55 million tons of ore reserves.
Graphite mines in North Korea are deeply buried and require underground mining. It is not easy for mines to increase production. In recent years, graphite production has stabilized at 30,000 tons; Madagascar graphite developers are Societe Miniere de la Grande Ile and Establissments Gallois. In 2008, graphite production was reduced to 5,000 tons. Ukrainian graphite deposits are located in Kirovograd Oblast. The graphite developer is Zavalivsky Grafitovy Kombinat. Ukraine’s graphite output is 75,000 to 10,000 tons; Sri Lanka has a world-famous vein. Graphite deposits are located in the west and southwest of Sri Lanka. Graphite veins are deeply buried and need to be mined underground. Bogala Graphite SriLanka is a subsidiary of German GK. The company imported equipment from the United Kingdom and newly equipped a graphite processing plant; the Czech Republic has two graphite developers, Coynur Graphite and Tyne Graphite. , Located in southern Bohemia, has been processing graphite products since the 17th century. Coinur Graphite Company mines and processes flake graphite. Tyne Graphite Company is a subsidiary of German GK Company. The company began to produce graphite products in 1965. In recent years, it has concentrated on the development of high-purity graphite, especially graphite products for batteries; The open-cut graphite mine operated by the Austrian company Grafitbergbau Kaiserberg is located in Kaiserberg and Trieben. The graphite processing plant operations include drying, grading, grinding and flotation as well as ultra-fine grinding. , The plant has a cryptocrystalline graphite flotation plant with a processing capacity of 30,000 tons. The company supplies fine graphite, large particle graphite, flake graphite and synthetic graphite; Timcal of Switzerland is the world's leading manufacturer of vertically integrated graphite. Graphite development is distributed in Canada, Europe and Asia, and product distribution agencies are distributed in South Africa, Australia, India, Malaysia and Thailand. The company has obtained 85% of the equity of the Inner Mongolia Baotou Jingyuan Graphite Company; Australia does not currently mine graphite, and it is possible to develop and produce graphite products in the future. Eagle Bay owns the Uley graphite deposit, which has a reserve of 3.77 million tons of carbon content of 7.4% and a reserve of 2.44 million tons of carbon content of 13.7%. There is a processing plant near the deposit. The plant ceased production in 1993, mainly Because the price of graphite is low in the international market. The Munglinup graphite deposit has large flake graphite, which is owned by a subsidiary of the lithium concentrate producer Gwalia. The graphite mine was mined from 1953 to 1956 due to the price of graphite. Production has been stopped for low-level reasons. It is estimated that the proven and inferred resources are 1.4 million tons, the average grade is 18.2%, and the depth is 55 meters.
Production and consumption overview
Affected by the recovery of the world economy on the graphite industry, the demand for graphite in the world has risen steadily in recent years. According to statistics from the United States Geological Survey (USGS), the world's major graphite producing countries in 2012 were China, India, Brazil, Canada, North Korea, Russia and the Czech Republic.
China has the largest output of 750,000 tons, accounting for 68% of the world's graphite output. The main exporters of natural graphite are China, Mexico, Canada, Brazil, and Madagascar. These countries export 97% of the world's graphite and account for 90% of graphite exports. Earthy graphite is mainly exported from Mexico, bulk graphite is exported from Sri Lanka, and China, Canada, and Madagascar export crystalline flake graphite.
With the advancement of heating technology and acid leaching technology, the purity of graphite that can be obtained is getting higher and higher, which opens up new applications of graphite in the field of high-tech technology. The innovation of purification technology has enabled graphite to have a wider range of applications in the fields of carbon composite materials, electronics industry, friction materials, and lubrication. The flexible graphite industry represented by graphite paper has a good market prospect, and the wide application of a large number of fuel cells has increased the amount of graphite.
China is a big producer and consumer of graphite, but it is not a strong graphite power. The current backward processing and purification technology in the graphite industry is extremely unbalanced in the country. In some areas, there are no graphite purification companies at all, and they are still selling graphite resources. For some high-tech natural graphite products, some need to be developed without graphite resources. National imports. The passive situation of selling medium carbon graphite to foreign businessmen at a cheap price and then buying high-purity graphite or graphite deep-processed products from foreign businessmen at a price dozens of times higher than our export prices still exists.
In order to transform my country from a big graphite country to a strong graphite country, it is necessary to take the graphite deep processing industry route and process graphite raw materials into graphite materials. In order to achieve this goal, we must first strengthen the research of graphite purification technology.
There is a problem
As early as the beginning of the 20th century, our country has begun to exploit graphite materials. Due to the large reserves of graphite in my country and the concentrated production areas, many convenient conditions are provided for the development of graphite in my country. At present, the development and utilization of new graphite technologies in my country has entered a stage of rapid development. Many new graphite products and new technologies have been highly utilized and developed. At present, there are mainly the following problems in the development of China's graphite industry:
(1) Graphite mining planning and overall planning are not in place
my country's graphite reserves rank second in the world, but due to the lack of unified investment and planning for graphite mining, there is no unified pricing and overall management of graphite in my country. The mining scale and output value are not high. The development and utilization of graphite mainly rely on the production and research and development of high-tech products to obtain greater added value and profits. In the current development and utilization of graphite in my country, the development and utilization of new products is in a disorderly state, and planning and overall planning cannot be combined. Combine to maximize benefits.
(2) Lack of channels for the development and utilization of graphite resources
Graphite resource is a non-renewable resource. Only by continuously increasing the development and utilization of graphite resources can we obtain greater product added value. At present, my country lacks channels for the development and utilization of graphite resources, and there is more primary production of graphite, but there are no channels for the development and utilization of high-tech materials. The most effective products in the market can be combined with production and processing to generate more maximum profits, both of which are indispensable. The combination of graphite resources and high-tech products will inevitably play an irreplaceable role in promoting the graphite industry. At present, the channels for combining the development and utilization of graphite resources in my country are relatively single, and there is no combination of market demand and product research and development.
(3) There is no unified plan for the establishment of graphite mining rights
Graphite is a kind of mining product, which seriously damages the mining environment. my country has not set up a unified plan for graphite mining rights, which has led to a large amount of disorderly mining and waste of resources. The mining of graphite minerals has the same characteristics as other mining. The initial investment in production capital is relatively large and the environmental impact is very serious. Only by carrying out a unified plan for graphite mining and reducing unnecessary repetitive inputs can the ratio of input to output be appropriately increased.
(4) The phenomenon of illegal mining is serious
Illegal mining in non-graphite mining areas is very serious. Graphite is a non-renewable resource, and the mining utilization rate determines the direct economic benefits of mining. The probability of reuse of pirated graphite ore is negligible. my country's current crackdown on the illegal mining of graphite is very strong, but in the face of high profits, many small mining owners still ignore it. Strengthening the crackdown on the illegal mining of graphite is of great significance to protecting the graphite mining industry.
The prerequisite of the graphite deep processing industry is purification. Graphite purification is a complex physical and chemical process. The purification methods mainly include flotation, alkaline acid, hydrofluoric acid, chlorinated roasting, and high-temperature methods.
Flotation
Flotation is a commonly used and important beneficiation method. Graphite has good natural floatability. Basically all graphite can be purified by flotation method. In order to protect graphite flakes, graphite flotation mostly adopts a multi-stage process. The graphite flotation collector generally uses kerosene with a dosage of 100-200g/t, and the foaming agent generally uses terpineol oil or butyl ether oil with a dosage of 50-250g/t.
The value and application of large flake graphite are much greater than that of fine flake graphite, and it cannot be recovered once damaged. Protecting the large scales of graphite in graphite beneficiation is a problem that cannot be ignored in the process of beneficiation. Because graphite has good natural floatability, the flotation method can increase the grade of graphite to 80% to 90%, even up to about 95%. The biggest advantage of this method is the one with the lowest energy consumption and reagent consumption and the lowest cost among all purification schemes. However, the silicate minerals and the compounds of potassium, calcium, sodium, magnesium, aluminum and other elements contained in the graphite flakes in a very fine state cannot be dissociated by the method of grinding, and it is not conducive to protecting the graphite flakes. . Therefore, flotation is only the primary means of graphite purification. To obtain high-carbon graphite with a carbon content of more than 99%, other methods must be used to purify it.
Alkaline Acid Method
The alkali-acid method includes two reaction processes: alkali melting process and acid leaching process. The alkali melting process uses the molten alkali and the acidic impurities in the graphite to undergo a chemical reaction under high temperature conditions, especially silicon-containing impurities (such as silicates, aluminosilicates, quartz, etc.) to generate soluble salts, and then After washing to remove impurities, the purity of graphite can be improved. The basic principle of the acid leaching process is to use acid to react with metal oxide impurities, which do not react with alkali during the alkali melting process. The metal oxide is converted into soluble salt, and then it is separated from graphite by washing. The combination of alkali fusion and acid leaching has a good effect on graphite purification.
A variety of alkaline substances can remove graphite impurities. The stronger the alkaline, the better the purification effect. The alkaline acid method mostly uses NaOH, which has a small melting point and strong alkalinity. The acid used in the acid leaching process can be HCl, H 2 SO 4 , HNO 3 or a mixture of them. Among them, HCl is more used.
For some graphite with high silicon content, the alkali fusion method to purify graphite can also realize the comprehensive recovery and utilization of silicon. The solution after the alkali fusion acid leaching is acidic, and the silicon impurities in the solution are converted into silicic acid. The silicic acid can be extracted by adding a certain amount of alum, and then calcined at a high temperature of 900° C. to obtain pure silica.
The alkaline-acid method is the most widely used method in the industrial production of graphite purification in my country. It has the characteristics of less one-time investment, higher product quality, and strong adaptability, as well as the advantages of simple equipment and strong versatility. The disadvantage is the need for high-temperature calcination, large energy consumption, long process flow, serious equipment corrosion, large loss of graphite and serious wastewater pollution. Therefore, comprehensive utilization technologies such as the use of graphite purification wastewater to prepare polychloroaluminum silicate are very important.
Hydrofluoric acid method
Hydrofluoric acid is a strong acid that can react with almost any impurities in graphite. Graphite has good acid resistance, especially hydrofluoric acid, which determines that graphite can be purified with hydrofluoric acid. The main process of the hydrofluoric acid method is the mixing of graphite and hydrofluoric acid. The hydrofluoric acid reacts with impurities for a period of time to produce soluble substances or volatiles, which are washed to remove impurities, dehydrated and dried to obtain purified graphite.
Hydrofluoric acid reacts with Ca, Mg, Fe and other metal oxides to form a precipitate, The generated H 2 SiF 6 dissolves in the solution, and can remove impurities such as Ca, Mg, and Fe. Hydrofluoric acid is highly toxic and seriously pollutes the environment. Cooperating with other acids to purify graphite can effectively reduce the amount of hydrofluoric acid. Hydrofluoric acid purification of graphite has the advantages of simple process flow, high product quality, relatively low cost, and little impact on the performance of graphite products. However, hydrofluoric acid is highly toxic, and safety protection measures must be taken during use. The waste water produced must be treated before it can be discharged, otherwise it will cause serious pollution to the environment.
Chlorination roasting method
The chlorination roasting method is to mix graphite with a certain reducing agent, and roast it at a high temperature under a specific equipment and atmosphere. The valuable metals in the material are transformed into metal chlorides in the gas or condensed phase, and they are separated from the rest of the components to make the graphite Purification process.
Impurities in graphite can be decomposed into oxides with higher melting and boiling points under high temperature conditions, such as SiO 2 , Al 2 O 3 , Fe 2 O 3 , CaO, and MgO. These oxides react with chlorine gas at a certain high temperature and atmosphere, and the metal oxides react with the chlorine gas to form chlorides with a lower boiling point. Therefore, at a lower temperature, these chlorides can be vaporized and escaped to achieve separation from the graphite, so that the graphite can be purified.
The advantages of the chlorinated roasting method are energy saving, high purification efficiency (>98%), and high recovery rate. However, there are also problems such as poisonous chlorine, severe corrosiveness and serious environmental pollution. The purity of the graphite produced in the process is limited, and the process stability is not good, which affects the application of the chlorination method in actual production, and it needs to be further improved and improved.
High temperature purification method
The melting point of graphite is 3850℃±50℃, which is one of the substances with the highest boiling point in nature, which is much higher than the boiling point of impurity silicate. Using their melting and boiling point difference, the graphite is placed in a graphitized graphite crucible, in a certain atmosphere, heated to 2700 ℃ with specific instruments and equipment, the impurities can be vaporized and escaped from the graphite to achieve the effect of purification. . This technology can purify graphite to more than 99.99%.
There are many factors influencing the purification of graphite by high-temperature method: ① The impurity content of graphite raw materials has the greatest impact on the effect of high-temperature purification. The impurity content of the raw materials is different, the ash content of the product obtained is different, and the purification effect of graphite with high carbon content is better. Graphite with a carbon content of 99% or more after purification by flotation method or alkaline acid method is often used as raw material; ②The carbon content of graphite crucible is also an important factor affecting the purification effect. The ash content of the crucible is lower than that of graphite, which helps graphite The ash in the medium escapes; ③Using high current, the graphite heats up quickly, which is conducive to the purification of graphite. It is best to use the raw material of high-power electrode and treat it at a high temperature of 2800℃; ④The particle size of graphite also has a certain impact on the purification effect.
High-temperature purification of graphite, the product quality is high, the carbon content can reach more than 99.995%, this is the biggest feature of the high-temperature method, but at the same time, it consumes a lot of energy and requires extremely high equipment, which requires special design and large investment. Raw materials also have certain requirements. Only graphite used in high-tech fields such as national defense, aerospace, and nuclear industry can be purified by this method.
application
Graphite can be used to produce refractory materials, conductive materials, wear resistant materials, lubricants, high temperature resistant sealing materials, corrosion resistant materials, heat insulation materials, adsorption materials, friction materials and radiation protection materials, etc. These materials are widely used in metallurgy, petrochemical industry , Machinery industry, electronics industry, nuclear industry and national defense, etc.
Refractory
In the iron and steel industry, graphite refractories are used for refractory linings of electric arc blast furnaces and oxygen converters, and refractory linings of ladle. Graphite refractories are mainly integral casting materials, magnesia carbon bricks and aluminum graphite refractories. Graphite is also used as a film-forming material for powder metallurgy and metal casting. Graphite powder is added to molten steel to increase the carbon content of steel, so that high-carbon steel has many excellent properties.
Conductive material
In the electrical industry, it is used to make electrodes, brushes, carbon rods, carbon tubes, mercury positive current devices, graphite washers, telephone parts, and coatings for television picture tubes.
Wear-resistant lubricating material
Graphite is often used as a lubricant in the machinery industry. Lubricating oil can not be used under high-speed, high-temperature, and high-pressure conditions, while graphite wear-resistant materials can work at high sliding speeds at a temperature of -200 to 2000°C without lubricating oil. Many equipment that transports corrosive media widely use graphite materials to make piston cups, seals and bearings. They do not need to add lubricating oil when they are running. Graphite emulsion is also a good lubricant for many metal processing (wire drawing, pipe drawing).
Corrosion-resistant materials
Specially processed graphite, which has the characteristics of corrosion resistance, good thermal conductivity, and low permeability, is widely used in the production of heat exchangers, reaction tanks, condensers, combustion towers, absorption towers, coolers, heaters, and filters , Pump equipment. It is widely used in petrochemical industry, hydrometallurgy, acid-base production, synthetic fiber, papermaking and other industrial sectors, which can save a lot of metal materials.
High temperature metallurgical materials
Because graphite has a small thermal expansion coefficient and can withstand rapid cold and rapid heat changes, it can be used as a glass mold. After graphite is used, ferrous metals can obtain castings with precise dimensions, high surface finish and high yield, and can be used without processing or a little processing. Save a lot of metal. The production of cemented carbide and other powder metallurgy processes usually use graphite materials to make porcelain boats for compression molds and sintering. Single crystal silicon crystal growth crucibles, regional refining vessels, support fixtures, induction heaters, etc. are all made of high-purity graphite. In addition, graphite can also be used for vacuum smelting graphite insulation boards and bases, high-temperature resistance furnace tubes and other components.
Atomic Energy and Defense Industry
Graphite has a good neutron moderator used in atomic reactors. Uranium-graphite reactors are currently the most widely used atomic reactors. The deceleration material in the nuclear reactor used as power should have a high melting point, stability, and corrosion resistance. Graphite can fully meet the above requirements. The purity of graphite used in atomic reactors is very high, and the impurity content should not exceed tens of ppm. In particular, the boron content should be less than 0.5 ppm. In the national defense industry, graphite is also used to make solid fuel rocket nozzles, missile nose cones, parts of aerospace equipment, heat insulation materials and radiation protection materials.
(1) Graphite can also prevent boiler fouling. Tests by related units have shown that adding a certain amount of graphite powder (approximately 4 to 5 grams per ton of water) can prevent boiler surface fouling. In addition, graphite coating on metal chimneys, roofs, bridges, and pipes can prevent corrosion and rust.
(2) Graphite gradually replaced copper as the material of choice for EDM electrodes.
(3) Graphite deep-processed products are added to plastic products and rubber products to prevent plastic products and rubber products from generating static electricity. Many industrial products need to have anti-static and electromagnetic radiation shielding functions. Graphite products have both functions. Graphite Applications in plastic products, rubber products and other related industrial products will also increase.
In addition, graphite is also a polishing agent and rust inhibitor for glass and papermaking in light industry, and is an indispensable raw material for the manufacture of pencils, ink, black paint, ink, synthetic diamonds, and diamonds. It is a very good energy-saving and environmentally friendly material, and the United States has used it as a car battery. With the development of modern science and technology and industry, the application field of graphite is still expanding. It has become an important raw material for new composite materials in the high-tech field, and plays an important role in the national economy.
Graphite deep processing
status quo
Due to the continuous growth of my country's metallurgical steel industry and the rapid development of lithium-ion batteries in the world, the demand for graphite raw materials has been stimulated. At the same time, the increasing attention of the industry and the government to the role of graphite strategic resources has caused the price of graphite mineral products to rise rapidly, turning 20 Over the years, the price of other mineral products has been rising, but the price of graphite has been constantly falling. This has not only improved the efficiency of the graphite industry, but also caused some social funds to continue to flow into the graphite industry. This great situation is certainly a great opportunity for the development of the graphite industry, but if we blindly expand the amount of mining and selection without scientific planning and reasonable guidance, the "black storm" of random mining in the late 1980s may occur again. Caused significant losses to the development of the graphite industry.
my country is a big country of graphite resources, but for a long time, the low-tech level of production and price competition in the graphite industry has been severely insufficient in capital and technology input. The main production of low-end products of raw ore and beneficiation has caused the industry to be in a long-term downturn. This situation has caused my country's graphite deep processing technology and products to lag behind developed countries, but the resource-rich countries are weak in deep processing. This is very incompatible with the rapid development of my country's economy and technology. At present and for a long period of time in the future, it is a promising undertaking to protect and scientifically use graphite, a precious strategic resource, and develop graphite deep processing technology and products.
The development of deep processing technology of flake graphite in my country has a certain foundation. The national scientific and technological research and support plans of the Ministry of Science and Technology from the "Eighth Five-Year" to the "Eleventh Five-Year Plan" are included in the non-metallic mines and western development projects, and have made progress in deep processing technology. Obvious results. At present, there are already a number of good-efficiency (scale) graphite deep processing enterprises in China. The largest flexible graphite enterprises are mainly distributed in Jiangsu and Zhejiang regions, and battery material companies such as anode materials are mainly distributed in Shenzhen and other Pearl River Delta and Yangtze River Delta regions.
The deep processing technology of microcrystalline graphite in my country is basically blank. Recent studies have found that due to the small size of the microcrystalline graphite (≤1μm), there are many disorderly accumulations of microcrystals in each graphite particle, making the particles appear isotropic. This makes it an excellent raw material for anode materials and isotropic graphite for lithium-ion batteries (especially power batteries), and has important application value in high-tech fields such as new energy, nuclear energy, and military industry. Tsinghua University has carried out original scientific research and development in this area, and is cooperating with related companies to build production lines for microcrystalline graphite purification and deep processing products.
Product form
(1) High purity graphite
It is mainly used as a stabilizer in military and industrial materials and industrial catalysis in other industries. It has complete crystallization and very good thermal conductivity.
(2) Isostatically pressed graphite
Isostatic graphite is an extended product of high-purity graphite. It is mainly processed from high-purity graphite. It has the characteristics of high-purity graphite, and has the main characteristics of low thermal expansion rate and excellent thermal conductivity after heating.
(3) Expandable graphite
Expandable graphite mainly selects naturally arranged natural flake graphite, which is mainly an interlayer compound after acidic oxidation treatment. In addition to the advantages of high temperature resistance and high heat resistance, the body has the advantages of increasing the expandability of graphite.
(4) Graphite fluoride
Graphite fluoride is a new type of graphite product that combines performance and benefit. Graphite functional material with high added value and unique quality, widely used in many fields. Mainly used in the fields of battery raw materials and solid lubricants.
Because graphite fluoride has low surface energy and high electrical activity, it can be used as an active material for batteries and is widely used in primary lithium-ion batteries. Graphite fluoride is mainly mixed with lithium or lithium-containing organic solvents to make high-performance lithium batteries. In addition to being used as a positive electrode material for lithium batteries, graphite fluoride can also be used as a positive electrode material for high-energy density magnesium batteries and aluminum ion batteries.
In addition, compared with other solid lubricants, fluorinated graphite has better lubricating properties and is almost unaffected by the environment, such as high temperature, high pressure, and corrosive environments. It can exhibit excellent performance. Due to its stable properties and excellent lubricating properties, it can be used as a lubricant and lubricant additive for mechanical equipment and sealing materials that operate in harsh environments.
(5) Colloidal graphite
Colloidal graphite mainly uses the characteristics of uniform graphite film formation in addition to ensuring excellent electrical conductivity and thermal conductivity, and is mainly used in the field of electrostatic film formation.
(6) Graphene
Graphene is a two-dimensional material with a thickness of one atom. It is mainly used as a bulletproof material and conductive agent in the military field.
Direction of development
Focus on deep processing, realize some important engineering projects, build a complete industrial chain, and guide the healthy and scientific development of the graphite industry. The first is the transformation of outdated technical equipment; the second is the current hot technology products in the development of carbon graphite materials, such as the industrialization and intensification of lithium-ion battery anode materials, isotropic graphite, and high thermal conductivity graphite.
(1) Renewal of graphite mining and beneficiation technology and equipment
my country's graphite mining and beneficiation technology and equipment have basically not improved since the 1960s, and are far behind other minerals in terms of energy consumption and mineral recovery. Graphite mining and beneficiation technology and equipment are simpler than other minerals, but due to the long-term low industrial benefits and lack of funds, they have not been updated. A powerful mineral design research institute combined with mining and dressing enterprises, introducing advanced mining and dressing technology and equipment from other minerals, designing and constructing advanced graphite mining and dressing production lines, in terms of energy consumption, recovery rate, large scale protection, water conservation and utilization, and tailings Technical and economic indicators such as mine treatment have been significantly improved. After successful operation, it will be promoted in the industry, and advanced technical and economic indicators will be used as indicators for industry access and elimination of outdated technical equipment.
(2) Building an advanced large-scale graphite purification production line
China already has environmentally friendly and energy-saving advanced acid-base purification and energy-saving high-temperature purification technologies, government guidance, industry-university-research integration, and construction of different types of large-scale graphite purification production lines based on resource characteristics. Strictly restrict the use of hydrofluoric acid that seriously pollutes the environment in chemical purification.
(3) Industrial scale of natural graphite anode materials for lithium batteries and research and development of anode materials for power and energy storage batteries
There have been companies in China that prepare anode materials after spheroidization of flake graphite, such as Beterui, etc.; Tsinghua University and other institutions have the technology to prepare anode materials from microcrystalline graphite, but the industrial scale and product quality cannot meet the requirements of lithium-ion batteries. Rapidly evolving demand. Spherical graphite produced in large quantities in China is mainly exported to Japan and South Korea for foreign companies to produce high-quality anode materials. Relying on resources, scale the industry of natural graphite lithium-ion battery anode materials, and develop different quality anode materials to meet the needs of different grades of batteries to serialize products; develop safe and long-life natural graphite power and energy storage battery anodes material. With the rapid growth in demand for lithium-ion batteries, the market prospects for anode materials are very broad.
(4) Industrialization of natural graphite-based isotropic graphite
Isotropic graphite is widely used in nuclear energy, silicon crystal preparation, EDM, continuous steel casting, aerospace and other fields. It is a high-end product and strategic material of carbon materials. Two thirds of the isotropic graphite currently needed in our country depends on imports. The traditional technology to prepare isotropic graphite is complicated and costly. The microcrystalline graphite mineral particles themselves are isotropic, which is a good raw material for preparing isotropic graphite, and can simplify the process and reduce the cost. Industrial size samples have been prepared, and the isotropic parameter reaches 1.04 (the most demanding nuclear Graphite 1.05); after the spheroidization of flake graphite, it also has the potential to prepare isotropic graphite. Tsinghua University and others have their own original patented technology and are cooperating with enterprises to implement industrialization.
(5) Natural graphite-based high thermal conductivity material
The miniaturization of electronic equipment requires higher and higher integration of electronic devices, making heat dissipation a key technology in the IT industry, and there is an increasing demand for lightweight and high thermal conductivity materials. Flexible graphite has been widely used in LED displays and many electronic products as a soaking and thermal conductive material; using the excellent thermal conductivity of natural graphite, high-end thermal conductive materials with a thermal conductivity equal to or higher than that of copper and a density of only 1/4 of copper are prepared. Wuhan Science and Technology Universities, Shanxi Coal Chemical Research Institute, Tsinghua University, etc. have related technologies. It is recommended to combine production, education and research to realize industrialization.
(6) Serialized research on flexible graphite products
The production of flexible graphite in my country already has a certain scale, and there are many cooperations with foreign advanced enterprises. However, the products in my country are mostly low-end products, and the varieties and specifications are less than 1/5 of those of foreign countries. It is recommended to combine production, education, research and use, develop high-end products based on usage requirements, improve varieties and specifications, and make them serialized and standardized.
(7) Industrialization of expanded graphite environmental protection materials
There have been a lot of research results on the adsorption and treatment effect of expanded graphite on water pollution. Expanded graphite is far more effective and economical than ordinary activated carbon in the treatment of oil and organic pollution in water bodies. However, expanded graphite is inconvenient to transport and needs to be prepared on site. The preparation, use, recycling, and regeneration of expanded graphite environmentally friendly materials have certain technical difficulties. In addition, in the past, environmental protection was not paid enough attention to, and expanded graphite environmentally friendly materials have not been industrialized. The 18th National Congress of the Communist Party of China proposed to build a beautiful China and increase environmental protection efforts, making the industrialization of expanded graphite environmentally friendly materials possible. Now that the technical basis for the industrialization of expanded graphite environmental protection materials is in place, this project can be carried out from two technical levels.
In areas where water pollution companies, such as steel, chemical, printing and dyeing, and food companies are concentrated, build a service network for the preparation, use, recycling, and regeneration of expanded graphite environmentally friendly materials. The use of expanded graphite environmental protection materials and other treatment methods will greatly improve the degree and benefits of water pollution treatment. In response to the increasingly frequent occurrence of oil and organic water pollution incidents, the construction of comprehensive water area environmental protection special ships, combining traditional fencing, suction and other treatment methods with the high-efficiency oil absorption performance of expanded graphite to improve pollution control capabilities.






 Account not verified
Account not verified